One of the most important topics on the mind of piping and process engineers is how to prevent corrosion under insulation (CUI) within their mechanical systems. Many of our clients have expressed an urgent need to understand this process and what steps they can take to proactively address this potential hazard in their plants.
A basic understanding of the science behind corrosion helps equip one to make more informed decisions about how various insulation products interact with metals to either induce or inhibit corrosion.
What is corrosion? It is a chemical process that gradually breaks down materials as electrons are exchanged and new molecules formed. The process requires liquid water and air or an electrolyte solution. With ferrous (iron-based) metals, corrosion is known as rust, which is the weakening of iron due to oxidation of its atoms. Here is a short video that illustrates how corrosion occurs at a molecular level:Video on Corrosion Basics
There are two main types of corrosion:
- Galvanic corrosion
- Non-galvanic corrosion
Galvanic corrosion happens when two different types of metals are exposed to an electrolyte solution (a liquid that is able to conduct electricity, like salt water). This exposure catalyzes a process where the metals exchange electrons through the electrolyte solution. As a result, one metal erodes while the other grows. Galvanic corrosion frequently happens in marine environments because salt water is a highly effective electrolyte solution.
Bear in mind, not all metals are equally susceptible to galvanic corrosion. There are a handful of metals called noble metals, such as gold, silver, and platinum, that are resistant to corrosion.
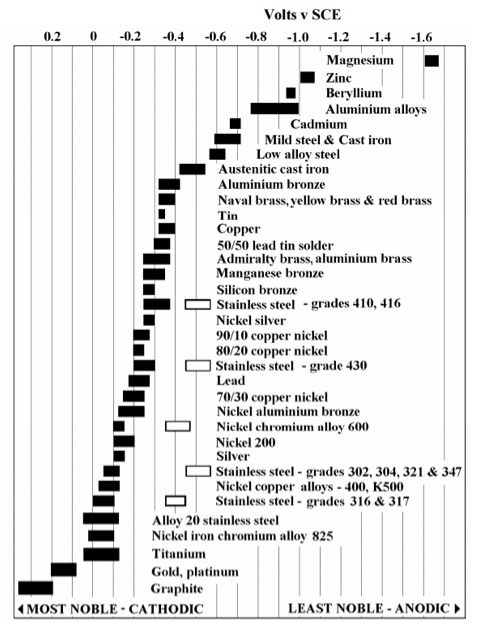
Chart 1: Nobility of Metals
Atlas Tech Note No. 7: Galvanic Steel. Page 3. (2010, August). Retrieved January 12, 2016, from http://www.atlassteels.com.au/documents/TN7-Galvanic_Corrosion_rev_Aug_2...
Other metals such as zinc or aluminum rank very low on the nobility scale and as such are highly susceptible to corrosion (see Chart 1).
One method to prevent galvanic corrosion is called hot-dipped galvanizing, and it uses liquid zinc to coat carbon steel (mild steel). This creates galvanized steel, and over time, the zinc will erode, protecting the steel until the zinc coating has eroded enough to fail.
This may lead some to ask, why don’t we just use galvanized steel or other zinc-rich coatings to provide protection against CUI in mid to high-temperature industrial process piping and vessels? Well, there is a specific reason, which is explained a document written by the National Association of Corrosion Engineers called NACE SP0198-10 Section 4.3.5:
“Inorganic zinc-rich coating shall not be used by itself under thermal insulation in the 50 to 175°C (120°F to 350°F) service temperature range. Zinc provides inadequate corrosion resistance in closed, sometimes wet, environments. At elevated temperatures greater than 60°C, the zinc may undergo a galvanic reversal whereby the zinc becomes cathodic to the carbon steel.”
Essentially, this means that at elevated temperatures, the zinc and the steel may reverse roles, where the steel begins to corrode while the zinc remains intact.
The second form of corrosion is known as non-galvanic corrosion, and it is an oxidation process. Oxidation simply refers to the loss of electrons. When certain metals are exposed to air and water, they begin to lose electrons, and this subsequently causes erosion. While non-galvanic corrosion requires air and water to take place, it does not require two different types of metal. This is the primary mechanism that causes CUI.
The primary method for preventing non-galvanic corrosion is to introduce a passivation layer to the system. A passivation layer is a thin coating that makes the underlying metal less susceptible to corrosion by physically blocking contact with air and water. This is the same concept as painting your car or applying a highly cross-linked epoxy phenolic or thermal spray aluminum (TSA) coating on industrial process piping and vessels.
When it comes to insulating industrial process pipes and vessels, understanding which corrosion mechanism is at play is essential to choosing the correct corrosion-prevention method. As non-galvanic corrosion is the primary culprit of CUI, choosing an insulation that can directly combat it is crucial.
Many choose to use hydrophobic insulations and coatings to prevent water from entering the system, thus halting non-galvanic corrosion; however these defense mechanisms can burn off at high temperatures or fail over time. As a result, relying solely on the hydrophobic insulation as the primary means of CUI prevention can be risky. Fortunately, there are certain types of industrial insulations that contain a unique chemical composition that actually inhibits corrosion under insulation by forming a passivation layer when water penetrates the system.
All pre-formed calcium silicate and expanded perlite insulations manufactured in the United States feature a proprietary corrosion inhibiting package that forms a passivation layer on the pipe systems. We are too conservative to suggest eliminating anti-CUI coatings altogether, but this chemistry package is a proven belt-and-suspenders approach that provides back up protection should the coating fail due to application errors, burn-out or aging.
The chemistry package is called XOX™, and it has been integral to all Johns Manville Thermo-12 Gold® and Sproule® WR-1200 since 2002. XOX-treated insulations demonstrate the lowest 3rd party tested ASTM C1617 mass loss corrosion rates of any industrial insulations on the market. Here’s exactly how it works to inhibit corrosion:
- Passivation layer
- Liquid water penetrates the system through a failure in the jacketing
- Water dissolves silicate anions contained in the insulation (the anions dissolve at varying rates over time, enabling XOX to provide long-lasting protection)
- These silicate anions settle on the surface of the metal
- With heat and time silica gel bonds with iron ions to form an inorganic iron silicate gel
- Natural passivation layer prevents electrolytes in water from contacting metal surface
- pH buffering effect
- Anions from the silicates react with and neutralize any acidic (low ph) components in the water
- Anions from the silicates strip off hydrogen atoms of various acids, thereby neutralizing them
- The pH of absorbed water averages around 10 which is above the corrosion range of <7
Understanding how corrosion happens is the first step in developing a defense that can slow or halt the chemical process that causes it. In the end, XOX is a solution that uses the chemistry that causes corrosion to actively protect the system – changing an attack on the system into a defense mechanism. XOX capitalizes on corrosion chemistry to create lasting corrosion protection that is unaffected by temperature or time.
If you would like more information about corrosion under insulation and the best methods to combat it, then we encourage you to attend our live webinar on January 20, 2016.
